This article, part 3 in a six-part series on the lymphatic system, discusses its role in protecting the body from invasive pathogens and toxins
Abstract
The lymphatic system plays an important role in providing immune responses to harmful micro-organisms and toxins that enter the body. This article, the third in a six-part series on the system, discusses its main functions in providing immunity.
Citation: Nigam Y, Knight J (2020) The lymphatic system 3: its role in the immune system. Nursing Times [online]; 116: 12, 45-49.
Authors: Yamni Nigam is professor in biomedical science; John Knight is associate professor in biomedical science; both at the College of Human and Health Sciences, Swansea University.
- This article has been double-blind peer reviewed
- Scroll down to read the article or download a print-friendly PDF here (if the PDF fails to fully download please try again using a different browser)
- Click here to see other articles in this series
Introduction
Potential pathogens (micro-organisms capable of causing disease) are ubiquitous in the environment and can enter the body via the skin (through direct contact, particularly if the epidermis is injured through cuts, grazes or burns), the respiratory system (through inhalation), the gut (through ingestion) and the genito-urinary tract (through sex or the insertion of invasive devices such as catheters). Although each of these sites is protected by unique barriers and defences, some pathogens can breach these preliminary defences and enter the body.
The immune system comprises a range of cells – some basic and innate, others extremely specialised – to detect and remove pathogens from the body. The lymphatic system works alongside the immune system to destroy unwanted pathogens either locally and directly, or by alerting the whole body to the infection and helping to mount a wider systemic immune response.
The first two articles in this series discussed the role of lymph in supporting the cardiovascular system, and examined the organs and tissues that make up the lymphatic system. This article focuses on the immune function of the lymphatic system.
“The spleen is often considered to be a structurally larger version of a lymph node”
Immune cells of the lymphatic system
The immune system includes a vast range of distributed defence cells: these are the leucocytes (white blood cells). The more-basic innate immune leucocytes are the first responders, responsible for immediate and non-specific engagement with a pathogen; they include phagocytes (cells capable of engulfing and absorbing bacteria and other small cells and particles) such as macrophages and dendritic cells, which encounter and indiscriminately ‘eat’ unwanted microbes or infected cells.
If innate cells cannot deal with the pathogen, more-specialised cells known as lymphocytes need to be ‘introduced’ to a pathogen to recognise it as a threat, before they can launch an attack on it.
Macrophages
These large-cell phagocytes are derived from monocytes (large phagocytic white blood cells); they can be fixed in tissues or mobile in the blood. Macrophages are capable of reeling in microbes with their cytoplasmic extensions (pseudopods) and engulfing them; they are tough cells that survive well and can perform this function many times over. Macrophages are also able to trap antigens (small molecules found on the surface of all cells) and present them to other leucocytes of the immune system.
Healthy cells in the body contain self-antigens, which act as important flags to prevent the immune system from attacking the body’s own cells. Phagocytes recognise pathogens and the toxins they may produce as foreign bodies by the presence of their different (non-self) antigens; they engulf and sequester (capture/trap) these foreign pathogens, which are then rapidly killed by intracellular digestion.
Lymphocytes
Lymphocytes are sentinel cells of adaptive immunity; they make up 20-30% of circulating leucocytes and include B-lymphocytes and T-lymphocytes. Bone marrow harbours about 12% of the body’s lymphocytes, whereas the spleen and lymph nodes contain approximately 55% of resident lymphocytes; the remainder are found in other lymphatic organs and tissues. Free lymphocytes in the blood amount to only about 2% of the lymphocyte population (Pabst, 2018).
B-lymphocytes
B-lymphocyte cells (B-cells) are formed and mature in the bone marrow. Once released, they develop the ability to determine which antigens they should react to (immunocompetence) and which are harmless (self-tolerance). Mature B-cells colonise secondary lymphoid organs such as lymph nodes or the spleen and:
- Are responsible for humoral immunity (which acts via the humors – fluids such as blood or lymph);
- Combat pathogens by producing and dispatching antibodies.
Collectively called immunoglobulins (Ig), antibodies are among the most abundant protein components in the blood and an important part of the immune system (Alberts et al, 2015). As a naïve B-cell becomes fully mature, it can display thousands of membrane-bound antibodies on its surface, and each B-cell has its own unique set of these ready to identify and bind to a particular antigen. If a random encounter with a potentially pathogenic foreign antigen results in binding and triggering of any of these membrane antibodies, it activates the B-cell.
The B-cell rapidly clones itself, forming masses of B-cells, all with the same instructions for producing the antibody designed to fight that particular antigen. The majority of these cloned cells become B-plasma cells – large antibody-producing factories – while a small proportion are retained as memory B-cells, able to quickly mass produce the same antibodies again if that particular antigen is encountered in future. Plasma cells are capable of producing around 2,000 antibodies per second (Alberts et al, 2015). They are usually detectable in the humor (plasma) after 4-7 days and float freely in blood and lymph, binding to foreign antigens on the surface of the pathogen or to the toxin that triggered their formation.
The role of antibodies. Although they cannot directly destroy antigens or kill pathogens themselves, the action of antibodies can:
- Help to inhibit micro-organisms;
- Highlight them for detection and attack by other immune cells.
First, as antibodies bind to the foreign pathogens, they neutralise them by physically blocking binding sites on the pathogen so it cannot attach to tissue cells and cause disease.
Second, antibodies cause agglutination of pathogens as they can bind to more than one antigen simultaneously. Agglutinated pathogens clump and cannot move around as easily, so it is easier for macrophages to detect and phagocytose them, and for other more-specialised lymphocytes to kill them. This ‘coating’ of foreign cells by antibody molecules is known as opsonisation and makes the pathogen more attractive to circulating phagocytes.
Finally, when bound to their corresponding antigens, antibodies can activate a system of potent plasma enzymes of the complement system. This group of 20 or so proteins, once activated, forms a protein conglomerate – termed a membrane attack complex (MAC) – which attacks and ruptures pathogens’ membranes leading to cell lysis and death. The complement system can be activated by antigen-antibody complexes or recognition of bacteria.
T-lymphocytes
Pathogens are not always found in fluids; many become intracellular (invade the cells) where antibodies cannot reach. Fortunately, another branch of adaptive immunity can provide more direct cell-to-cell combat. This is cell-mediated immunity and is facilitated by the T-lymphocytes (T-cells), which are produced in the bone marrow and mature in the thymus gland (see part 2).
There are a few different types of T-cells, but the two main ones are:
- Helper T-cells;
- Cytotoxic T-cells.
They are able to locate compromised body cells (those that have been hijacked by pathogens or become cancerous). However, T-cells can only do this if they are ‘told’ to do so by other cells: during phagocytosis, phagocytes ingest and break up pathogens into numerous tiny molecules. Some of these molecules are moved onto grooved proteins, termed major histocompatibility complexes (MHCs), on the surface of the phagocyte and displayed there. Cells that can do this are known as antigen-presenting cells (APCs).
T-cells are unable to recognise whole antigens, but they can recognise parts of them when they are displayed on an APC. Just as naïve B-cells carry antibodies on their surface for one specific antigen, naïve helper T-cells have receptors that will only bind to one specific combination of MHC and antigen. If these cells meet an APC displaying the correct MHC-antigen match, the helper T-cells will bind to it. Once bound, the helper T-cell is activated and, with the help of chemical messengers (cytokines), quickly clones itself to produce many more helper T-cells and cytokines.
Some of these cytokines now activate cytotoxic T-cells, which can kill marked, rogue cells by releasing potent enzymes that puncture the target cell membrane, resulting in cell death. Cytokines are important molecules in the immune system – in particular, interleukin 1 plays a significant role in initiating the inflammatory process, acting as the major endogenous pyrogen to promote fever during infection, as well as helping B-cells to rapidly undergo proliferation and clonal expansion.
Helper T-cells also play a crucial role in helping B-cells to become fully activated to produce antibodies. They do this by ‘checking’ that the antigen presented by B-cells is one the body needs to react to and destroy. In fact, helper T-cells play the most vital role in the provision of immunity.
Dendritic cells. These cells are effective APCs that migrate from the bone marrow into peripheral tissues. They are a pivotal link between the innate immune system and the adaptive immune system. So-called because their surface membrane looks similar to the tree-like dendrites of neurones, dendritic cells are key in activating T-cells by presenting microbial antigens to them. Their wispy extensions and surface pattern recognition receptors recognise common features of many microbial pathogens, making them efficient antigen catchers. Dendritic cells bind to and phagocytose pathogens and, once activated and displaying phagocytosed antigens, migrate from tissue into lymphatic vessels.
The role of lymph and lymph nodes
As potential sites of infection are infiltrated by a dense network of lymphatic capillaries (see part 1), it is also inevitable that pathogens and/or their antigens will end up circulating in the lymphatic system. Parts 1 and part 2 of this series discussed the importance of tissue drainage and the transport of lymph back into the circulatory system to ensure homoeostasis. While lymph is circulating around the body, it passes through various ‘checkpoint’ sites of the lymphatic system; these sites include lymph nodes, the spleen and various types of mucosa-associated lymphoid tissue (MALT) (Fig 1). The lymph nodes, in particular, play a major role in trapping foreign material.

Immunity at the lymph nodes
Approximately 600-700 lymph nodes are situated in clusters around the body in lymphatic vessels; they range from about 1-2mm to 2cm in size and are often palpable in the neck, armpit and groin.
These tightly packed balls of lymphoid cells and protein primarily act to:
- Monitor lymph arriving at each node for pathogens that may have entered the system;
- Attempt to eliminate them before they can cause any damage to the body.
The lymph nodes play host to a series of complex cellular interactions that typically lead to activation of residing cells, the lymphocytes and macrophages; depending on what they detect at the lymph node, they can activate locally in the node or activate immunity systemically outside the node.
The structure of lymph nodes is discussed in part 2. T-cells are mainly gathered in the paracortex, whereas B-cells cluster primarily in the follicles of the outer cortex. Lymph (carrying invader antigens, either free or bound to dendritic cells) enters nodes via an afferent lymphatic vessel. Macrophages lining the lymphatic sinuses of the lymph node may transfer free antigens to T- and B-cells, which are equipped with receptors that are each capable of recognising specific foreign antigens.
Antigens usually reach the lymph node carried by APCs, which home into the paracortical region and stimulate antigen-specific T-cells (Fig 2). If a responsive T-cell encounters a specific antigen on the antigen-presenting dendritic cell, it becomes activated to now proliferate and differentiate into effector T-cells, resulting in enlargement of this T-cell zone. Activated T-cells leave the lymph node via the efferent lymphatic vessel.
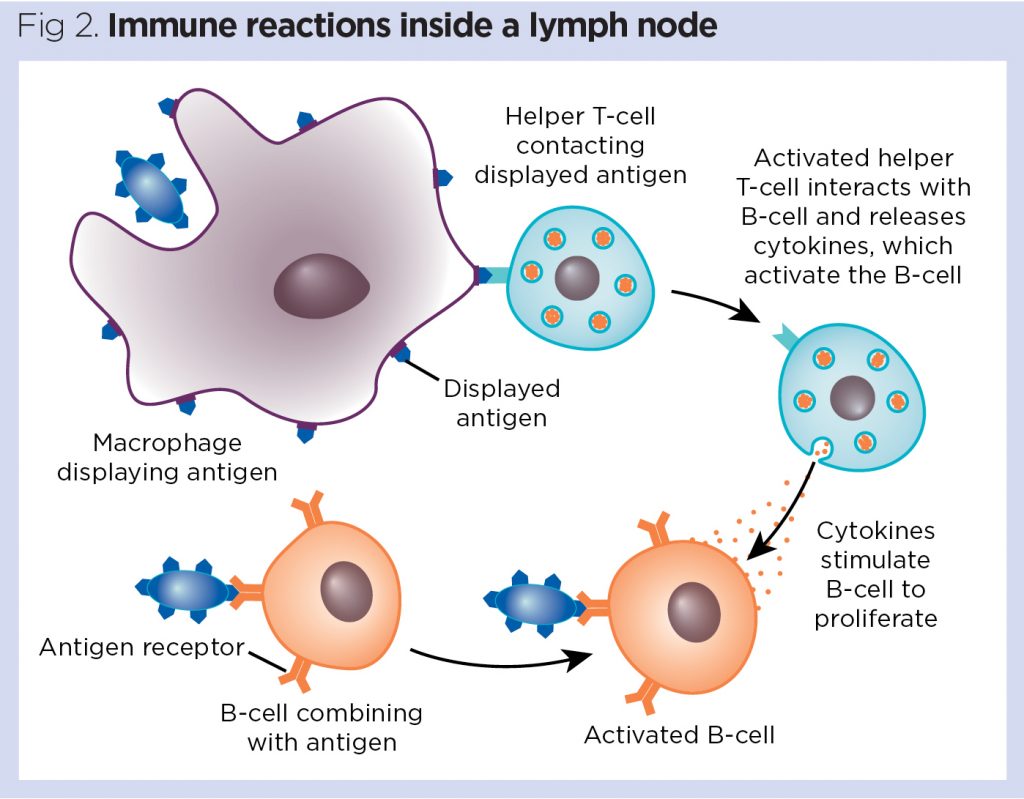
Stimulation of B-cells in the primary follicles of the lymph node results in the development of secondary follicles with the formation of germinal centres. Antigen-activated B-cells migrate to the medullary cords, where they differentiate into antibody-producing plasma cells and begin production of specific antibodies (Fig 2). Antibodies produced in the course of a humoral immune response also leave the lymph node predominantly via the efferent lymphatic vessel to systemically fight the infection.
The immune system at a lymph node works in synergy to help defeat pathogens; indeed, it has been shown that follicular B-cells can also undergo phagocytosis to acquire antigen to show helper T-cells (Martínez-Riaño et al, 2018). Following B-cell activation, antigens are sequestered and memory B-cells home into the germinal centres of the lymph node.
Lymph node swelling
Following the events described above, some noticeable effects may become apparent. As the antibody-producing B-cells begin to proliferate in the germinal centres and T-cells robustly clone into effector T-cells, the scaffolding meshwork of the lymph node (fibroblastic reticular cell network) relaxes and becomes elastic and flexible to support the large number of expanding cells. The affected lymph nodes begin to enlarge and may become palpable and tender. Health professionals use this fact in localising and tracing the origins of infections at the time of diagnosis (Table 1). As B-cell action subsides and the T-cells leave the lymph node to travel around the body to fight the infection, the lymph node meshwork returns to its original size.

Immunity in the spleen
The spleen plays an important role in mounting a targeted response to invading pathogens. It is often considered to be a structurally larger version of a lymph node. The red pulp that makes up 75% of spleen tissue has a major job of destroying old red blood cells and breaking down haem, while the white pulp is fundamental to the adaptive immune response. In the white pulp, the spleen’s anatomical structure supports both B- and T-cell activation.
Surrounding the central arteriole bringing blood into the white pulp is the periarterial lymphatic sheath (Fig 3), predominantly harbouring macrophages and T-cells. Around the sheath is the marginal zone, containing more macrophages and follicles, which are also fed via a blood capillary. Follicles contain naïve B-cells.

The white pulp can mount an immune response to foreign invaders arriving in the blood in a number of ways:
- Dendritic cells arriving at the spleen with a foreign antigen present it to T-cells in the periarterial lymphatic sheath, which become activated. T-cells now activate B-cells in follicles to become antibody-producing plasma cells, either in the red or white pulp. Antibodies leave the follicles to travel widely through the systemic circulation;
- A pathogen such as a virus enters the spleen in the blood. Follicular B-cells come into contact with the virus and present viral antigen to nearby T-cells. Both cells co-stimulate and activate each other. The B-cells produce antibody against the virus;
- Macrophages in the spleen can also pick up the foreign pathogen or antigen, presenting to T-cells which activate B-cells to produce plasma cells and antibody.
The spleen often enlarges when blood-borne infections are present, but splenic enlargement (splenomegaly) is also associated with other diseases; one example is liver failure, which would affect iron breakdown in the splenic red pulp.
Memory B-cells
Memory B-cells are able to persist in the body, maintaining memory for a given antigen for decades; they are most abundant in the spleen, making up 45% of the total B-cell population in this organ, but they also recirculate in the blood (Hauser and Höpken, 2015). In the case of an antigen first encountered in the spleen, the memory B-cells produced during the primary response tend to congregate in the splenic marginal zones, where blood-borne antigens may predominantly collect. In response to an antigen first encountered in a lymph node, some of the memory B-cells produced remain in the follicle of the lymph node and are ready to react rapidly if the antigen is ever conveyed again to the lymph node. However, other memory B-cells may leave the original lymph node and enter the blood, circulating among the body’s chain of lymph nodes and maintaining peripheral surveillance for the antigen (Mak et al, 2014).
Immunity offered by mucosal-associated lymphoid tissue
The spleen and lymph nodes are not the only lookout points of the body. Mucosa-associated lymphoid tissue is a form of diffuse lymphoid tissue, an arrangement of lymphoid cells and protein, found in mucous membranes outside the lymphatic vessels. It is strategically positioned at entry points of particularly sensitive tissue, such as the respiratory and gastrointestinal tracts, and includes:
- Gut-associated lymphoid tissue (GALT);
- Bronchus-associated lymphoid tissue (BALT);
- The paired palatine (visible at the rear of the throat), lingual (located at the root of the tongue) and the naso-pharyngeal tonsils (adenoids) (Fig 4).

The tonsils are the largest aggregates of lymphatic tissue located in the pharynx; together, they form a ring of tissue (Waldeyer’s tonsillar ring) that is ideally situated to remove pathogens from the air or food before they can enter the lungs or gastrointestinal tract. As a result of stimulation by pathogens here, sore throats and swollen tonsils are often a visible and painful sign of a viral or other infection.
One part of GALT, Peyer’s patches, are nodules of lymphoid tissue situated in the distal portion of the small intestine. Another, the appendix, situated at the entrance of the large intestine, contains lymphoid tissue that can destroy bacteria to prevent it breaching, or being absorbed by, the intestinal wall. In both GALT and BALT, mucosal epithelium is scattered with cells known as M-cells, which can trap antigens, small particles or entire micro-organisms and deliver them from the lumen to macrophages and dendritic cells beneath the epithelium. These, in turn, activate B-cells and T-cells lying under the epithelium in the mucosal tissue, which proceed to deal with them through the humoral and cellular means previously described.
Conclusion
In summary, the lymphatic system forms a major part of the immune system, defending the body against infections and harmful bacteria or viruses. However, there are circumstances when the immune system becomes overactive and begins to react to substances that are normally harmless. These substances – allergens such as dust or pollen – can cause an allergic reaction.
Part 4 of this series on the lymphatic system focuses on allergies and the more severe allergic responses, which could lead to anaphylaxis or life-threatening anaphylactic shock.
Key points
- The lymphatic system plays an important role in the immune system
- It produces a range of cells that detect and kill invading pathogenic micro-organisms and toxins
- Phagocytes can deal with many pathogens, but others need a more specialised response from lymphocytes
- Circulating lymph transports some of these cells around the body to encounter pathogens and toxins
- Various ‘checkpoints’ in the lymphatic system can raise a response when lymph containing pathogens passes through them
Also in this series
- The lymphatic system 1: structure, function and oedema
- The lymphatic system 2: structure and function of the lymphoid organs
- The lymphatic system 4: allergies, anaphylaxis and anaphylactic shock
- The lymphatic system 5: vaccinations and immunological memory
- The lymphatic system 6: the history and function of immunotherapies
Alberts B et al (2015) Molecular Biology of the Cell. Garland Science.
Hauser AE, Höpken UE (2015) B-cell localization and migration in health and disease. In: Alt FW et al (eds). Molecular Biology of B Cells. Academic Press.
Mak TW et al (2014) T-cell development, activation and effector functions. In: Primer to the Immune Response. Academic Cell.
Martínez-Riaño A et al (2018) Antigen phagocytosis by B cells is required for a potent humoral response. EMBO Reports; 19: 9, e46016.
Pabst R (2018) The bone marrow is not only a primary lymphoid organ: the critical role for T-lymphocyte migration and housing of long-term memory plasma cells. European Journal of Immunology; 48: 7, 1096-1100.
 Nursing Times Resources for the nursing profession
Nursing Times Resources for the nursing profession 

Have your say
or a new account to join the discussion.